Patients expect pain relief from an acute analgesic to be rapid and effective. In order to achieve this and to further improve analgesics with the proven drug ibuprofen, there are essentially two approaches: combining it with other substances such as the co-analgesic caffeine or formulating ibuprofen as the lysine salt, with the aim of accelerating its absorption into the bloodstream (which results in faster relief of pain).
 Nederland
NederlandPharmacokinetics of ibuprofen: comparison of the fixed combination of ibuprofen plus caffeine with ibuprofen lysinate or ibuprofen acid
Results of two single-dose studies (randomised, single centre, crossover, open-label)
The superior efficacy of ibuprofen (400 mg) plus caffeine (100 mg) compared with ibuprofen (400 mg) alone has been clearly established [1]. Two clinical trials have also investigated the pharmacokinetics of ibuprofen from the combination and those of ibuprofen lysinate, both on an empty stomach (>10h fast) as well as after ingestion of a standard breakfast [2].
Methods
Two clinical studies were conducted, each with 36 subjects. In the first study (NCT01879371) the pharmacokinetics of ibuprofen from the fixed-dose combination with caffeine were compared with those of ibuprofen (as acid or lysinate) from two comparator preparations. The study preparations were taken after >10 h fasting, during which only the drinking of water was permitted.
In the second study (NCT02629354) the pharmacokinetics of ibuprofen from the fixed-dose combination were compared with those of ibuprofen (as lysinate) after ingestion of a standard breakfast.
These single-dose studies were randomised, single-centre, crossover and open label. The key parameters measured were peak plasma concentrations (Cmax), time to reach them (tmax) and total exposure (AUC0–t). These parameters were also measured for caffeine. Analytical and statistical methods corresponded to the usual standards [2].
Results
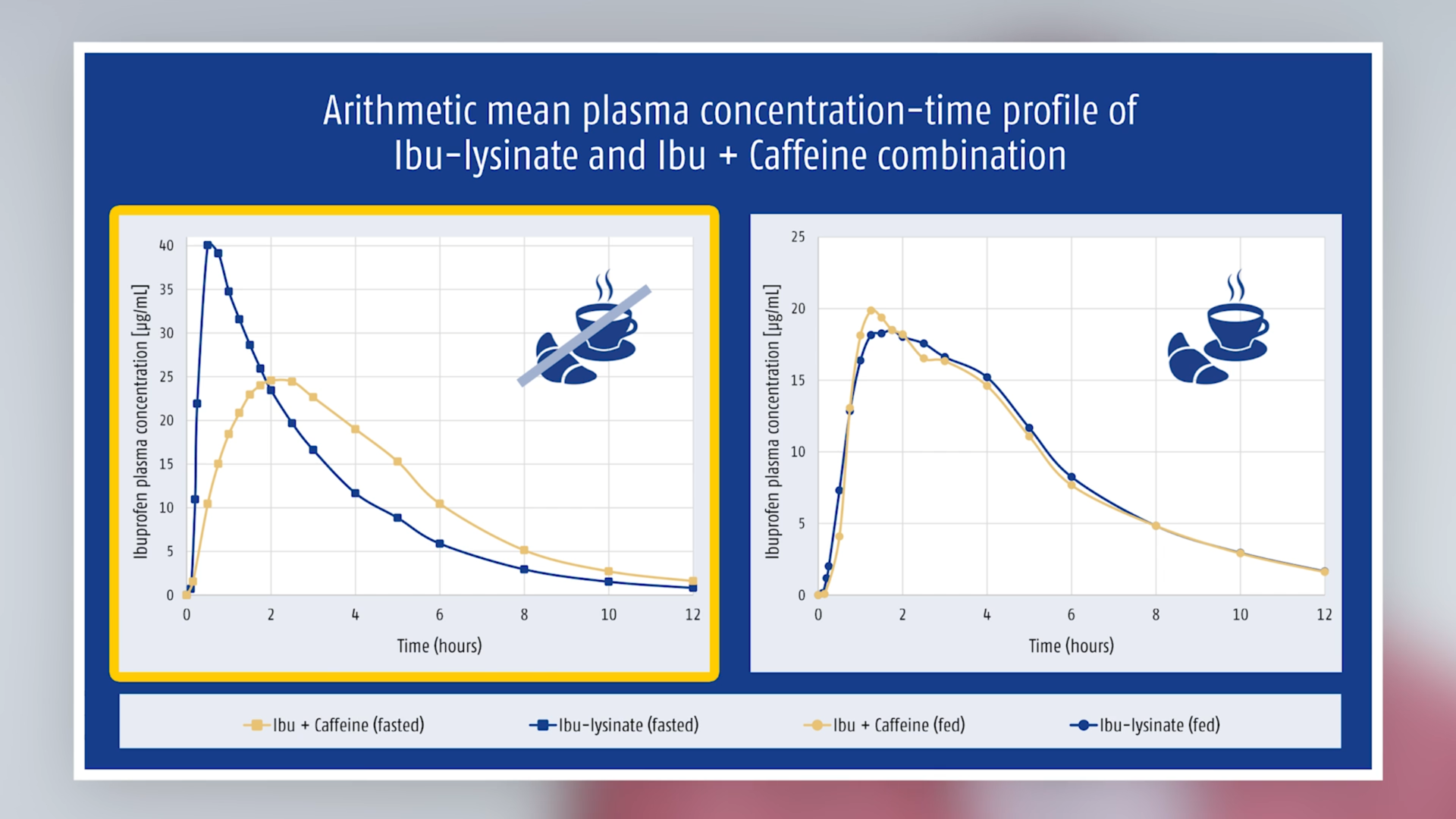
Ingestion on a fasted stomach showed the expected results: ibuprofen as lysinate was absorbed faster than ibuprofen acid (from the fixed-dose combination or from the ibuprofen-only preparation); and despite comparable exposure, peak plasma concentrations were higher (see Table).
A different picture emerged if taken after a standard breakfast, i.e. on a non-fasted stomach: under these conditions, ibuprofen plasma concentrations from the combination were higher than from ibuprofen lysinate and the time to reach peak plasma concentrations was shorter (see Table and Figure 1). The difference between fasted/non-fasted states was also significantly smaller than with ibuprofen lysinate (Figure 2).
Fig. 1 Arithmetic mean plasma concentration-time profiles of ibuprofen and caffeine from the fixed-dose combination
Fig. 2 Arithmetic mean plasma concentration-time profiles of ibuprofen (as lysinate)
Dicussion and conclusions
According to the definition of the European drug regulatory authorities, the term “fasting” means that the test person in a study does not eat anything for at least 8 hours before and 4 hours after taking the investigational product, drinks only non-carbonated water, and for one hour before and after ingestion is not allowed to drink anything at all. The investigational products must be taken with exactly 150 ml of water [3]. Under these standardised conditions, it is possible to compare different studies quite well; however, these conditions are very remote from everyday life.
Analgesics are usually not taken under conditions that correspond to the standard requirements of the regulatory authorities for “fasting”. In order to achieve this state, someone would have to take an analgesic before breakfast (and only if the evening meal hadn’t been eaten too late!) and would also have to skip breakfast itself. These are the only conditions when a more rapid absorption (and higher peak plasma concentrations) of ibuprofen as lysinate compared with ibuprofen acid is proven. Older data showed that after a standard breakfast, ibuprofen lysinate and acid have comparable pharmacokinetic properties, i.e. ibuprofen lysinate has no advantage [4]. The more recent publication shows that ibuprofen from the combination with caffeine is absorbed from a non-fasted stomach more rapidly and attains higher peak plasma concentrations than ibuprofen lysinate [2].
Literature
- Weiser et al. Efficacy and safety of a fixed-dose combination of ibuprofen and caffeine in the management of moderate to severe dental pain after third molar extraction. Eur J Pain. 2018;22(1):28–38. doi: 10.1002/ejp.1068.
- Weiser et al. Pharmacokinetic Properties of Ibuprofen (IBU) From the Fixed-Dose Combination IBU/Caffeine (400/100 mg; FDC) in Comparison With 400 mg IBU as Acid or Lysinate Under Fasted and Fed Conditions-Data From 2 Single-Center, Single-Dose, Randomized Crossover Studies in Healthy Volunteers. Clin Pharmacol Drug Dev. 2019;8(6):742–753. doi: 10.1002/cpdd.672.
- EMA GUIDELINE ON THE INVESTIGATION OF BIOEQUIVALENCE, 2010 https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed 05.12.2019)
- Klüglich et al. Ibuprofen extrudate, a novel, rapidly dissolving ibuprofen formulation: relative bioavailability compared to ibuprofen lysinate and regular ibuprofen, and food effect on all formulations. J Clin Pharmacol. 2015;45(9):1055–61.
Conflict of interest: T. Weiser is an employee of Sanofi.
Disclosure: Publication funded by Sanofi Aventis Deutschland GmbH.