Great Britain
Great BritainCaffeine – stimulating and aromatic: but safe!
The European Commission asked the European Food Safety Authority (EFSA) to compile this scientific Opinion on the safety of caffeine. The assessment was based on data from 39 surveys in 22 European countries with a total of 66,531 participants. Some of the questions addressed by the report were as follows:
- What amounts of caffeine consumed, either short-term, or habitually and over long periods of time, have no harmful effects on the health of healthy adults?
- What effects does habitual caffeine consumption have on relevant subgroups such as pregnant or lactating women, children or adolescents?
Caffeine in foods and drinks
Caffeine is the most frequently consumed pharmacological substance worldwide [1]. Parts of plants such as coffee and cocoa beans, tea leaves, guarana berries and kola nuts naturally contain caffeine. Synthetic caffeine is added to other foods and beverages such as soft drinks, confectionery or “energy drinks”. If one wants to take a look at daily caffeine intake, then consumption of a wide variety of foodstuffs needs to be considered.
It is generally assumed that one cup of coffee contains about 150 mg of caffeine. However, on closer inspection it is found that the caffeine content varies enormously and is unpredictable and non-reproducible. For example, the caffeine content ranges from 30 to 1780 mg/cup with brewed coffee and from 250 to 2140 mg/cup in the case of espresso. The highly variable caffeine concentration in ready-to-drink coffee beverages depends on the manufacturing process, the type of coffee beans used and the preparation method (e.g. filter coffee, espresso) [2].
Effects of caffeine consumption on healthy adults
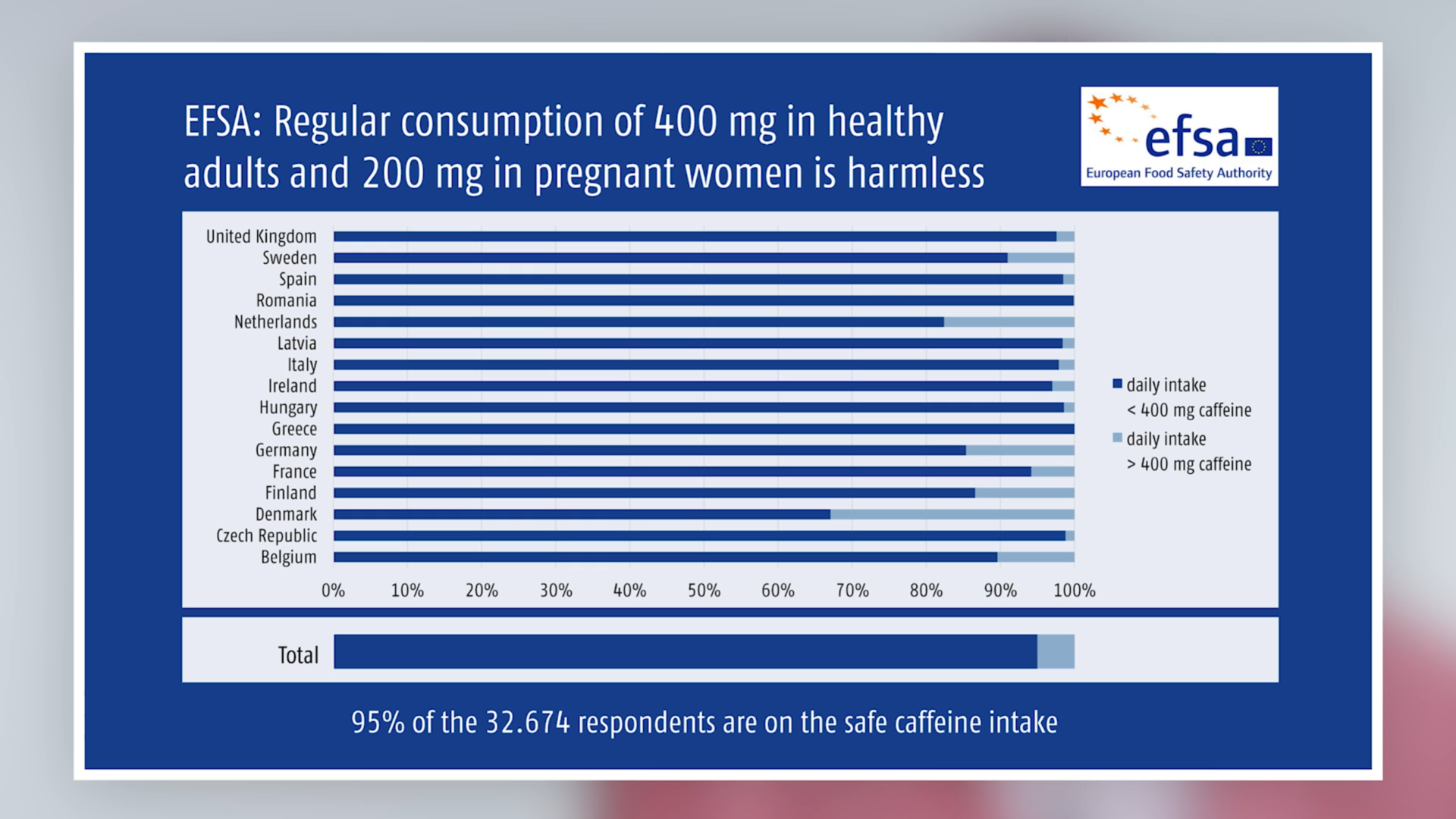
According to the Opinion, a single dose of 200 mg does not pose a health risk for healthy adults although in the Panel’s view, short-term effects on the central nervous system such as sleep disturbances are possible. (A more detailed overview of the effects of caffeine on sleep can be found in [2]). Habitual caffeine intake of up to 400 mg per day (about 5.7 mg/kg body weight daily for an adult weighing 70 kg) also appears to have no harmful effects in relation to acute toxicity, bone status, cardiovascular health, cancer risk or male fertility.
According to the EFSA study, the average caffeine consumption of adults (18 to 75 years) is between 37 and 319 mg/day (95% of adults consume less than about 100–700 mg/day). On the basis of these figures, the report rated caffeine consumption in this population group as safe.
Effects of caffeine consumption on relevant subgroups of the general population
Pregnant women: After evaluating the available data, the Opinion assessed the regular intake of up to 200 mg caffeine daily during pregnancy as harmless to the foetus. The average daily caffeine intake calculated from the available sources amounted to 109 mg per day (95% of the women consumed less than 206 mg per day).
Lactating women: An intake of up to 200 mg caffeine per day by the lactating mother results in a daily caffeine intake of the breast-fed infant of 0.3 mg/kg body weight. A dose-finding study on caffeine observed no adverse effects in the majority of infants with a ten-fold higher dose of 3 mg/kg body weight. Data on caffeine consumption in this population subgroup are rare and average 31 mg per day. 95% of breast-feeding women consumed less than 97 mg per day.
Children and adolescents: The European Food Safety Authority considered that the information available for this population subgroup on the relationship between caffeine intake and effects on health was insufficient to enable a safe caffeine intake to be derived.
Summary: The Panel on Dietetic Products, Nutrition and Allergies (NDA) of the EFSA considered the following amounts of caffeine, distributed over the day, as safe: 400 mg for healthy adults, 200 mg for pregnant and lactating women. The good news: despite the highly variable amounts of caffeine in foods and drinks, the EFSA considered the habitual consumption in 95% of those questioned did not give rise to health concerns and is safe.
Literature
- Römpp online: Entry on caffeine. Georg Thieme Verlag, Stuttgart.
- Waizenegger J, Castriglia S, Winkler G, Schneider R, Ruge W, Kersting M, Alexy U and Lachenmeier DW. Caffeine exposure in children and adolescents consuming ready-to-drink coffee products. Journal of Caffeine Research, 1. 2012;200–205.
- EFSA Panel on Dietetic Products, Nutrition an Allergies (NDA). Scientific Opinion on the safety of caffeine. European Food Safety Authority, Parma, Italy, 2015.
Conflict of interest: T. Weiser is an employee of Sanofi.
Disclosure: Medical writing and publication funded by Sanofi Aventis Deutschland GmbH.